Contents
Scope of the problem
Selected Causes of Tinnitus
I type
a) Pulsating: Pulsatile tinnitus
I type
a) Pulsating: Aneurism is the most dangerous condition
I type
a) Pulsating: Mitral insufficiency
I type
a) Pulsating: Temporo-mandibular joint sounds
b) Non-pulsating or periodical: Vessel sclerosis
b) Non-pulsating or periodical, Otosclerosis
II type
Subjective mascable – sensory neural hearing decrease (peripheral)
Methods important to establish type II tinnitus
Management
III type
Subjective non-mascable (with hyperacusia), mostly central
IV type
Slow brainstem degeneration syndrome
Conclusion
Scope of the problem
Tinnitus affects up to 40% of the population in Western countries at least temporarily. One to three percent of the general populations report a significant reduction in their quality of life due to their tinnitus, for example, through its effect on sleep and mood. It is assumed that tinnitus is a result of maladaptive cortical plasticity. Among 222.1±3.4 million American adults, 21.4±3.4 million (9.6±0.3%) experienced tinnitus in the past 12 months. Among tinnitus sufferers, 27.0% had symptoms greater than 15 years; 36.0% had near constant symptoms.
Higher rates of tinnitus were reported in those with consistent work (OR 3.3; CI: 2.9-3.7) and recreational time (OR 2.6; CI: 2.3-2.9) loud noise exposure. Years of work-related noise exposure correlated with increasing prevalence of tinnitus r=0.130 (95% CI, 0.103 to 0.157). 7.2% reported their tinnitus as a “big” or “very big” problem versus 41.6% reporting it as a “small” problem. Only 49.4% had discussed their tinnitus with a physician, where medications were the most frequently discussed recommendation (45.5%). Other interventions, such as hearing aids (9.2%), masking devices (4.9%), and cognitive-behavioral therapy (0.2%) were less frequently discussed.
A systematic review included all adult population studies reporting the prevalence of tinnitus from January 1980 to July 2015. The search being done in five databases (Embase, Medline, PsychInfo, CINAHL and Web of Science), using a combination of medical subject headings (MeSH) and relevant text words. Observational studies including cross-sectional studies were included, but studies estimating the incidence of tinnitus (e.g. cohort studies) were outside the scope of this systematic review.
The databases identified 875 papers and a further 16 were identified through manual searching. After duplicates were removed, 515 remained. On the basis of the title, abstract and full-text screening, 400, 48 and 27 papers respectively were removed. This left 40 papers, reporting 39 different studies, for data extraction. Sixteen countries were represented, with the majority of the studies from the European region (38.5%).
Publications since 2010 represented half of all included studies (48.7%). Overall prevalence figures for each study ranged from 5.1% to 42.7%. For the 12 studies that used the same definition of tinnitus, prevalence ranged from 11.9% to 30.3%. Twenty-six studies (66.7%) reported tinnitus prevalence by different age groups, and generally showed an increase in prevalence as age increases. Half of the studies reported tinnitus prevalence by gender. The pattern generally showed higher tinnitus prevalence among males than females.
There were 8 different types of definitions of tinnitus, the most common being “tinnitus lasting for more than five minutes at a time” (34.3%). Only seven studies gave any justification for the question that was used, or acknowledged the lack of standard questions for tinnitus. There is widespread inconsistency in defining and reporting tinnitus, leading to variability in prevalence estimates among studies.
Nearly half of the included studies had a high risk of bias and this limits the generalisability of prevalence estimates. In addition, the available prevalence data is heterogeneous thereby preventing the ability to pool the data and perform meta-analyses. Sources of heterogeneity include different diagnostic criteria, different age groups, different study focus and differences in reporting and analysis of the results. Heterogeneity thus made comparison across studies impracticable.
This was an observational study of 4.7 million residents of England under 85 years of age who were at risk for developing clinically significant tinnitus (sigT). SigT was defined by a discharge from hospital with a primary diagnosis of tinnitus, or a primary care recording of tinnitus with subsequent related medical follow-up within 28 days. The database used was the Clinical Practice Research Datalink and individual records were linked to additional data from the Hospital Episode Statistics.
The observational period was from January 1, 2002 to December 31, 2011. There were 14303 incident cases of sigT identified among 26.5 million person-years of observation. The incidence rate was 5.4 new cases of sigT per 10,000 person-years (95% confidence interval: 5.3 to 5.5). The incidence rate did not depend on gender but increased with age, peaking at 11.4 per 10000 in the age group 60 to 69 years.
The annual incidence rate of sigT increased from 4.5 per 10000 person-years in 2002 to 6.6 per 10000 person-years in 2011. The 10-year cumulative incidence of sigT was 58.4 cases (95% confidence interval: 57.4 to 59.4) per 10000 residents.
Nearly 324000 new cases of sigT are expected to occur in England between 2012 and 2021. Tinnitus presents a burden to the health care system that has been rising in recent years. Population-based studies provide crucial underpinning evidence; highlighting the need for further research to address issues around effective diagnosis and clinical management of this heterogeneous condition. That is why there are lots of attempts to produce tinnitus classification as it might be seen below.
Selected Causes of Tinnitus
Subjective tinnitus
- Otologic: hearing loss, Meniere’s disease, acoustic neuroma
- Ototoxic medications or substances
- Neurologic: multiple sclerosis, head injury
- Metabolic: thyroid disorder, hyperlipidemia, vitamin B12 deficiency
- Psychogenic: depression, anxiety, fibromyalgia
Objective tinnitus
- Vascular: arterial bruit, venous hum, arteriovenous malformation, vascular tumors
- Neurologic: Palatomyoclonus, idiopathic stapedial muscle spasm
- Patulous eustachian tube
Prof. Claussen and Prof. Shulman have differentiated four main groups of tinnitus types:
- Type I – bruit (objective),
- Type II – endogenous tinnitus (subjective mascable),
- Type III – exogenous tinnitus (subjective non-mascable),
- Type IV – slow brainstem degeneration syndrome.
In the literature there are a lot of bruit descriptions mostly from neuro and cardiosurgery.
I type
a) Pulsating: Pulsatile tinnitus
can have many causes. No prospective studies on this subject are available to date. Pulsatile tinnitus requires both a functional organ of hearing and a genuine, physical source of sound, which can, under certain conditions, even be objectified by an examiner. Pulsatile tinnitus can be classified by its site of generation as arterial, arteriovenous, or venous.
Typical arterial causes are arteriosclerosis, dissection, and fibromuscular dysplasia. Common causes at the arteriovenous junction include arteriovenous fistulae and highly vascularized skull base tumors. Common venous causes are intracranial hypertension and, as predisposing factors, anomalies and normal variants of the basal veins and sinuses. In the series of patients, pulsatile tinnitus was most often due to highly vascularized tumors of the temporal bone (16%), followed by venous normal variants and anomalies (14%) and vascular stenoses (9%).
Dural arteriovenous fistulae, inflammatory hyperemia, and intracranial hypertension were tied for fourth place (8% each). Management in most cases is surgical.
I type
a) Pulsating: Aneurism is the most dangerous condition
There are about 20,400 results in Google search showing results for pulsatile tinnitus brain aneurysm though the authors consider these cases to be rather rare. Pulsatile tinnitus is tinnitus that coincides with the patient’s heartbeat. Aneurysm of the internal carotid artery is known as a rare cause of pulsatile tinnitus and, in the main, aneurysms of the petrous portion have been reported as a cause of pulsatile tinnitus.
A 54-year-old woman visited tinnitus clinic because of pulsatile tinnitus on the right side of her head for several years. She had taken hypertensive medicine for 5 years, but her blood pressure was well controlled and she denied any trauma history to her neck or head. On physical examination, her tympanic membrane and external auditory canal showed normal appearance, and she did not present any neurological signs. Her tinnitus could not be auscultated around the neck and auricle, and did not disappear after putting digital pressure on her neck.
Audiologic examination showed mild sensorineural hearing loss of high-pitched tones on both sides. On imaging study, temporal bone computed tomography looked normal, but her brain magnetic resonance imaging (MRI) showed a 1.4-cm signal void in the paraclinoid region of the right ICA. For further evaluation, intra-arterial angiography was performed and confirmed a large saccular aneurysm arising from the posterior side of the paraclinoid segment of the ICA. Aneurysm is the indication for the urgent surgical management.
Another interesting case is reported by Conlin et al. A 67-year-old woman presented with a constant, pulsatile, ringing in her left ear that had been ongoing for 12 months. She had no history of head trauma, surgery, hypertension or cardiovascular disorders. The patient was in no distress and appeared to be otherwise healthy. Findings on otoscopy and examination of the cranial nerves were unremarkable. Given the patient’s history of unilateral, pulsatile tinnitus, we auscultated her skull and heard a soft, high-pitched bruit over her left mastoid process.
The bruit was more prominent when we asked the patient to turn her head to the left. We did not hear any such sounds over her neck, and the rest of the examination of her head and neck yielded normal results. A computed tomography (CT) scan of the head showed a prominence in our patient’s left cavernous sinus. There was no enhancing vascular mass and no evidence of herniation of the brainstem.
Magnetic resonance imaging showed a dural arteriovenous fistula on the patient’s left side, with prominent arterial supply from the left middle meningeal and left occipital arteries. There was also evidence of diffuse dural enhancement consistent with venous hypertension. Because we thought that the fistula might rupture and cause a cerebral hemorrhage, we referred her for urgent consultation with a neurosurgeon, who recommended a diagnostic catheter angiogram to document the anatomy of the fistula.
Treatment involved catheterassisted coiling of the fistula using a Guglielmi detachable coil. There were no complications during or after the procedure. The patient’s tinnitus resolved completely, and she has remained symptom-free for the past three years.
Radiological neurovisualisation has shown next reasons of the pulsating tinnitus. Tympanic cavity pathology, temporal bone pathology, e.g., otosclerosis, Paget disease, and Langerhans Cell Histiocytosis (LHC), paraganglioma, hypervascular metastasis or meningeoma, vascular channel dehiscence or variant, aberrant ICA or stapedial artery, vascular loops, neurovascular conflict, arteriovenous fistula.
I type
a) Pulsating: Mitral insufficiency
Other conditions that may be associated with pulsatile tinnitus include valvular heart disease, particular aortic stenosis and mitral regurgitation, as well as hyperdynamic state associated with anemia or thyrotoxicosis.
I type
a) Pulsating: Temporo-mandibular joint sounds
The most significant otologic symptoms, consisting of ear pain, tinnitus, dizziness, hearing loss and auricolar “fullness”, generally arise within the auditory system, often are associated with extra auricular disorders, particularly disorder of the temporo-mandibular joint. In this study a sample of 200 consecutive patients being examined who had experienced severe disabling symptom.
The patiens came to maxillofacial specialist assessment for temporomandibular disorder. Each patient was assessed by a detailed anamnestic and clinical temporomandibular joint examination and they are divided into five main groups according classification criteria established by Wilkes; tinnitus and subjective indicators of pain are evaluated.
The results of this study provide a close correlation between the joint pathology and otologic symptoms, particularly regarding tinnitus and balance disorders, and that this relationship is greater the more advanced is the stage of joint pathology. Moreover, this study shows that TMD-related tinnitus principally affects a younger population (average fifth decade of life) and mainly women (more than 2/3 of the cases). Such evidence suggests the existence of a specific tinnitus subtype that may be defined as “TMD-related somatosensory tinnitus”.
b) Non-pulsating or periodical: Vessel sclerosis
Many patients will have no radiologic abnormalities than can explain their tinnitus. On the other hand, many radiologic findings associated with tinnitus are also seen frequently in asymptomatic individuals. The correlation of imaging findings with clinical symptoms remains a critical step before suggesting that a particular radiologic finding may be responsible for a patient’s tinnitus. Also PET and SPECT might be useful (Fig. 1).
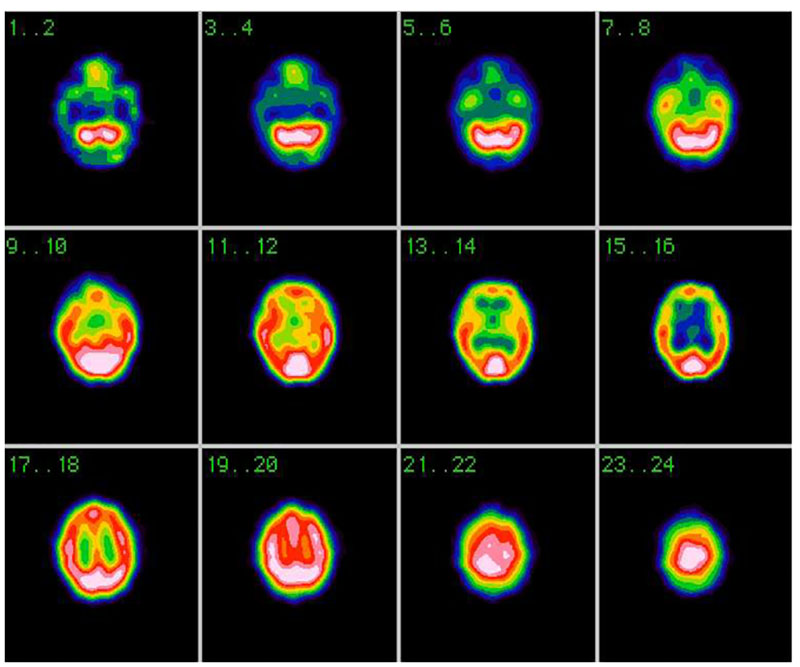
b) Non-pulsating or periodical, Otosclerosis
or otospongiosis is an idiopathic infiltrative process of the petrous temporal bone that may present with tinnitus. The most common type is fenestral otosclerosis (85%), which presents with conductive hearing loss. Cochlear or retrofenestral otosclerosis (15%) causes additional sensorineural hearing loss.
Chronic subjective tinnitus is a common feature of clinical otosclerosis, reported in ≤65% of patients. In early stages of otosclerosis, a radiolucent focus may be seen at the fissula ante fenestram, which is a cleft of fibrocartilaginous tissue located just anterior to the oval window. As the disease progresses, this lucency involves the margins of the oval and round windows and may even produce multiple radiolucent foci. Extension of the disease to the otic capsule may produce imaging signs of both fenestral and cochlear otosclerosis in the same patient.
Cochlear otosclerosis is characterized by low-attenuation foci around the basal turn of the cochlea, though some lesions may also involve the lateral walls of the IAC and the cochlear promontory. In severe otosclerosis, a low-attenuation ring sometimes surrounds the cochlea, known as the double-ring sign.
In the later healing phase, CT may show a “heaped-up” appearance along the margins of the oval and round windows and around the cochlea, indicating new bone formation. MR imaging is less sensitive for these findings, though T2-weighted images may show a high intraosseous signal intensity in the cochlear form of otosclerosis.
For diagnostic useful appears to be audiogram, which has typical features with difference of the air and bone conduction. Otosclerosis is often the result of the inflammation in the middle ear.
The efficacy of etidronate, a bisphosphonate, was assessed as a treatment for the inner ear symptoms of otosclerosis in a retrospective case review of 896 patients diagnosed with otosclerosis, with primary complaints of dizziness, hearing loss, tinnitus or Meniere’s syndrome. The diagnosis of otosclerosis was based on small-pixel computed tomography of the temporal bones. Of the 896 patients placed on an etidronate protocol, 545 were followed for more than six months and were analyzed.
The symptomatic response to etidronate, as well as audiologic and computerized rotary chair results were used in the assessment. Patients who were previously on sodium fluoride were separately analyzed. In this preliminary study etidronate appeared to be an effective treatment for the neurootologic symptoms of otosclerosis. Prospective blinded efficacy studies of the bisphosphonates in the treatment of otosclerosis should be undertaken.
II type
Subjective mascable – sensory neural hearing decrease (peripheral)
might have several reasons: disorder of the inner hair cells, disorder of outer hair cells, acoustic neuritis, acoustic neurinoma.
Auditory neuropathy is a hearing disorder characterized by normal function of outer hair cells, evidenced by intact cochlear microphonic (CM) potentials and otoacoustic emissions (OAEs), with absent or severely dyssynchronized auditory brainstem responses (ABRs). To determine if selective lesions of inner hair cells (IHCs) and auditory nerve fibers (ANFs) can account for these primary clinical features of auditory neuropathy, the physiological responses from chinchillas with large lesions of ANFs (about 85%) and IHCs (45% loss in the apical half of the cochlea; 73% in the basal half) being measured.
Distortion product OAEs and CM potentials were significantly enhanced, whereas summating potentials and compound action potentials (CAPs) were significantly reduced. CAP threshold was elevated by 7.5dB, but response synchrony was well preserved down to threshold levels of stimulation. Similarly, ABR threshold was elevated by 5.6 dB, but all waves were present and well synchronized down to threshold levels in all animals.
Thus, large lesions of IHCs and ANFs reduced response amplitudes but did not abolish or severely dys-synchronize CAPs or ABRs. Pathologies other than or in addition to ANF and IHC loss are likely to account for the evoked potential dyssynchrony that is a clinical hallmark of auditory neuropathy in humans.
Apoptosis, or controlled cell death, is a normal part of cellular lifespan. Cell death of cochlear hair cells causes deafness; an apoptotic process that is not well understood. Worldwide, 1.3 billion humans suffer some form of hearing loss, while 360 million suffer debilitating hearing loss as a direct result of the absence of these cochlear hair cells. Much is known about apoptosis in other systems and in other cell types thanks to studies done since the mid-20th century. Here is the review of current literature on apoptosis in general, and causes of deafness and cochlear hair cells loss as a result of apoptosis.
The family of B-cell lymphoma (Bcl) proteins are among the most studied and characterized. The review current literature on the Bcl2 and Bcl6 protein interactions in relation to apoptosis and their possible roles in vulnerability and survival of cochlear hair cells is important in future.
One of the most common complaints of adults visiting an audiology clinic is difficulty in understanding speech in the presence of background noise. Performance in noise is often attributed to sensorineural hearing loss.
However, there are many individuals with normal hearing who report difficulty hearing in background noise, which demonstrates how inadequate a typical audiometric evaluation is in evaluating the demands of speech comprehension in complex listening situations. It is also unclear which anatomical structures are responsible for our performance in background noise.
Discovering the roles of inner hair cells (IHCs), outer hair cells (OHCs), and auditory neurons in speech understanding in quiet and in the presence of background noise is currently a hot topic in the scientific community. Previous studies have suggested that Auditory Nerve (AN), may play a role in speech understanding in the presence of background noise, noise exposure has been shown to cause temporary damage to OHCs (as measured by Distortion Product Otoacoustic Emissions [DP-OAEs]) and permanent damage to the AN (measured by a decrease in the wave I amplitude of the Auditory Brainstem Response [ABR]).
The authors of these studies speculate that a decrease in AN activity would affect speech in noise performance.
However, measuring speech in noise among humans is a difficult task. The American Auditory Society and the American Academy of Audiology presented preliminary data in this realm at the 2016 annual meetings of the Association for Research in Otolaryngology, the American Auditory Society, and the American Academy of Audiology. The aim of our study was to investigate the roles that OHCs and the AN play in audition in quiet and performance in noise.
Participants included 40 English speaking adults (mean 45 years old) recruited from audiology clinic. All subjects underwent an audiological evaluation that included tympanometry, air and bone conduction thresholds, speech reception thresholds (SRT), and word recognition in quiet (WR-Q) using recorded NU6 word lists. These measures were performed for a single ear and stimuli were presented at 0, 10, 20, and 40 dB SL to control for hearing loss. OHC function was evaluated by measuring DP-OAE amplitudes and thresholds. AN function was measured by the amplitude, latency, and threshold of wave I of the ABR.
Preliminary findings revealed that those who exhibited better word recognition in noise were younger people with better hearing, more robust OHC function (both DPOAE amplitude and DPAOE threshold), and longer spiral ganglion absolute latencies and higher thresholds. Unlike animal studies, wave I amplitude failed to be a reliable metric of AN function in humans (ABR amplitudes are known to be notoriously variable in awake human subjects). Rather, AN latency and threshold produced repeatable measurements of AN function.
However, preliminary results of AN function were not what it had been expected. The people who performed poorer in background noise were found to exhibit a shorter wave I latency and a lower wave I threshold, which on the surface was the opposite of what being expected based on animal models.
However, this discrepancy is likely due to the dysfunction of the tuning properties of the OHCs and AN. In the aforementioned animal models, the experimental model exhibited OHC function but a decrease in wave I amplitude. Therefore, it appears that normal OHC function is required for the expected lower wave I amplitude that would also exhibit a longer wave I latency and higher wave I threshold. In current study, both OHCs and AN were abnormal in persons exhibiting poorer performance in background noise.
Based on other animal studies, OHC damage should result in a decrease in wave I latency and a lowering of wave I threshold, which is what might be seen see in human data. These preliminary results suggest that both OHCs and the AN contribute to speech recognition in the presence of background noise. However, OHC damage may play a primary role in hearing difficulties in this complex listening situation.
Methods important to establish type II tinnitus
Audiometry
might be subjective and objective. It allows revealing the decrease of hearing function in patient as well as exact frequencies impaired.
Middle ear muscle reflex (MEMR):
The MEMR tests how well the ear responds to loud sounds. In a healthy ear, loud sounds trigger a reflex and cause the muscles in the middle ear to contract. In a patient with pathology, loud sounds don’t trigger the reflex or much louder sounds are needed to trigger it.
Otoacoustic emission (OAE):
This test measures how well the outer hair cells in the cochlea function. It’s done when the patient is lying still or asleep, either naturally or through mild sedation. A tiny probe that contains a special microphone is placed in the ear canal, pulsing sounds are sent through it, and a machine measures what kind of echo the sound causes in the outer hair cells.
Auditory brainstem response (ABR):
This test measures whether the auditory nerve transmits sound from the inner ear to the lower part of the brain and how loud sounds have to be for the brain to detect them. If the brain is not receiving the information in a clear way, this test can show that. The audiologist places tiny earphones in the ear and sends sounds through them while electrodes are placed on the patient’s head to measure the brain activity.
Magnetic resonance imaging (MRI)
to see if the auditory nerve is present in both ears and if there are any inner ear abnormalities.
Genetic testing
to see if tinnitus is caused by a genetic condition, and if so, what treatments may be helpful.
Neurologic testing
by a neurologist to look for any other nerve-related problems. Multisensory evoked potentials are effective.
Speech and language testing
A child with tinnitus needs regular visits with a speech-language-phonologist, who will monitor speech and language development to make sure the patient is on track.
Case report: Patient О, male, 69 y.o., complaining of tinnitus and dizziness, Audiometry has shown bilateral hearing decrease, everyday noises have masking effect as it is seen at Fig. 2.
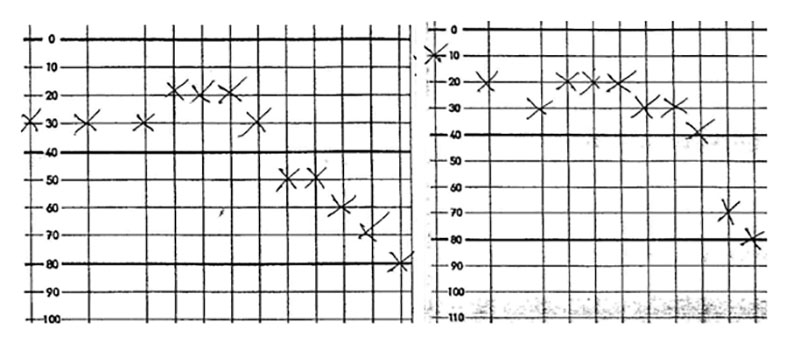
Pendular test has shown the decrease of vestbular nuclei activity at the brainstem level (Fig. 3).
Phonation crucially improved the vestibular function in this patient (Fig. 4).
Left picture – control study, right one – with phonation
This investigation not only explains the masking effect of external noises at this group of patients, but also provide easy-going test for which masking will be benefitial.
Management
Medications
In the acute cases (up to one year) sometimes it appears effective the combination of SH-group donators with nootrops, donators of nucleotids and activators of Crebbs cycle – according to our experience.
Cochlear implants
The Food and Drug Administration (FDA) estimated in 2012 that roughly 58,000 adults and 38,000 children use cochlear implants in the USA, compared to the more than 12 million Americans who wear hearing aids. To put those statistics into perspective, for every person wearing a cochlear implant, there are roughly 125 who wear hearing aids. Only half of 1 percent of those seeking treatment for sensorineural hearing loss will be fit with cochlear implants.
Californian ear institute proposes the next possibilities
to improve the life quality of the sensory-neural hearing loss sufferers. There seems to be two major factors in this process – the regeneration of the hair cell, and the reconnection of the hair cell to the nerve cells. It appears that when the hair cell is produced it secretes molecules called “trophic factors” which attract nerve fibers. When the hair cells are connected to the nerve cells, hearing is restored. The major obstacle in duplicating this process in humans is to generate new hair cells. This has to be done through a process of cell division. Recent experiments using animals have succeeded in promoting cell division within the inner ear using growth-promoting molecules. So far, similar molecules for human use have not been found, but at least the possibility of hair cell regeneration in mammals has been confirmed.
Transplants and gene therapy
Another approach that is investigated is to transplant hair cell precursors into the inner ear. Precursors are cells from which other cells are formed. Unfortunately, hair cell precursors have not yet been identified, but embryonic research is being done to identify which cells in the embryo develop into hair cells. Gene therapy is also being researched as a way to stimulate hair cell growth. Already, the genes responsible for stimulating precursor cells into hair cells had been identified. This method for regenerating hair cells within humans is being actively investigated with progress.
The Future
Damaged hair cells must be compensated for with the use of hearing aids or cochlear implants.
III type
Subjective non-mascable (with hyperacusia), mostly central
Many people with tinnitus also suffer from hyperacusis. Both clinical and basic scientific data indicate an overlap in pathophysiologic mechanisms. In order to further elucidate the interplay between tinnitus and hyperacusis the clinical and demographic characteristics of tinnitus patients with and without hyperacusis being compared by analyzing a large sample from an international tinnitus patient database.
The default dataset import [November 1st, 2012] from the Tinnitus Research Initiative [TRI] Database was used for analyses. Hyperacusis was defined by the question “Do sounds cause you pain or physical discomfort?” of the Tinnitus Sample Case History Questionnaire. Patients who answered this question with “yes” were contrasted with “no”-responders with respect to 41 variables. 935 [55%] out of 1713 patients were characterized as hyperacusis patients.
Hyperacusis in tinnitus was associated with younger age, higher tinnitus-related, mental and general distress; and higher rates of pain disorders and vertigo. In relation to objective audiological assessment patients with hyperacusis rated their subjective hearing function worse than those without hyperacusis. Similarly the tinnitus pitch was rated higher by hyperacusis patients in relation to the audiometrically determined tinnitus pitch.
Among patients with tinnitus and hyperacusis the tinnitus was more frequently modulated by external noise and somatic maneuvers, i.e., exposure to environmental sounds and head and neck movements change the tinnitus percept. Our findings suggest that the comorbidity of hyperacusis is a useful criterion for defining a subtype of tinnitus which is characterized by greater need of treatment. The higher sensitivity to auditory, somatosensory and vestibular input confirms the notion of an overactivation of an unspecific hypervigilance network in tinnitus patients with hyperacusis.
Prof. Shulman and Prof. Claussen have proved that some types of tinnitus appeared to be related to vestibular damage. Therefore, the documentation might be done using phonation procedure with vestibular tests: posturography, cranio-corpography or nystagmography. While in the cases of II type tinnitus phonation improves the performance, in the cases of the III type – the performance is impaired!
Blockers of H1 histaminic receptors, Ca channels and muscarinic blockers appeared to be effective, especially in the persons, who obtained contusions during rocket and fusil explosions (Ukrainian experience).
IV type
Slow brainstem degeneration syndrome
Etiology: old age, head trauma, poisoning, neural infections. Manifestation: dizziness, emotional disturbances, cognitive impairment, tinnitus and headaches.
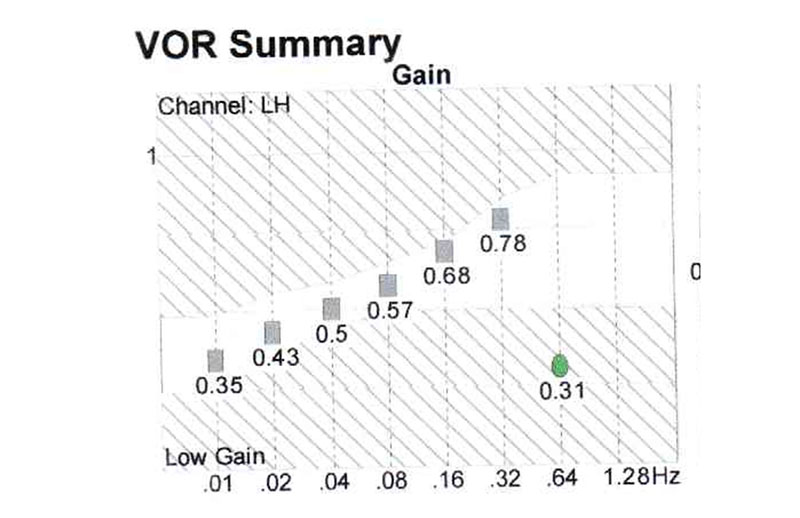
Case report: Patient H, female, 80 y.o., complaining of dizziness, nictophobia, discoordination, tinnitus and headaches. At pendular test serious decrease of gain at all the frequencies (Fig. 5), this study has revealed the decrease of the activity of the whole vestibular system.
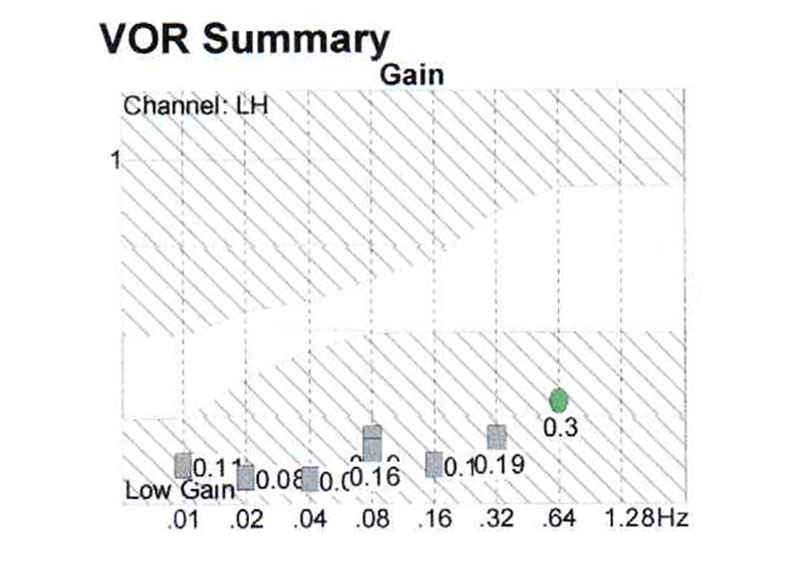
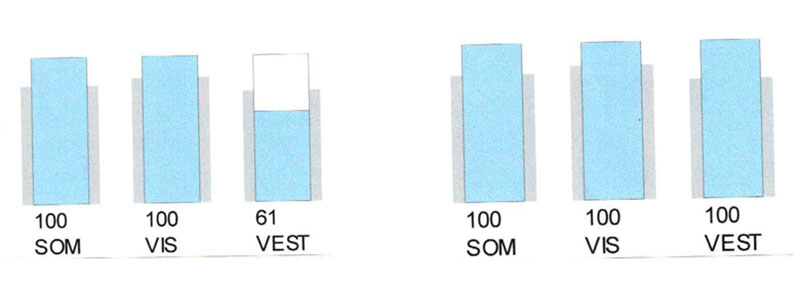
Phonation causes in this patient, like in the III type tinnitus, the impairment of the balance function, documented by posturography (Fig. 6).
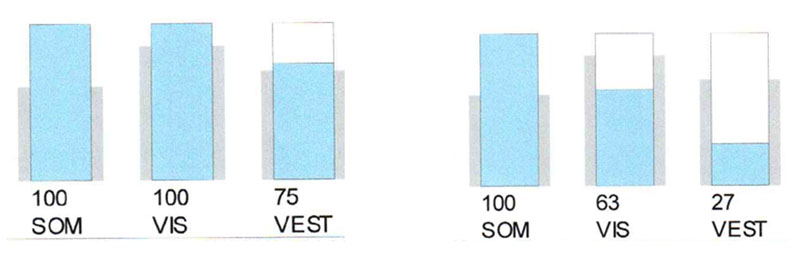
Management of such patients is enouph difficult, they need improvement of protein synthesis, nootrops, metabolism correction.
Conclusion
We need to consider the type of tinnitus to improve its management.
